Every oceanographer I know who is studying the controversial idea of coaxing the ocean to absorb extra carbon dioxide from the atmosphere remembers the moment they decided to start this contentious work. For me, it came during the Pacific Northwest heat wave of June 2021, which sent temperatures soaring above 49 degrees Celsius (120 degrees Fahrenheit) and set boreal forests ablaze.
I had spent years studying ocean circulation and Earth’s carbon cycle but not marine carbon dioxide removal (mCDR)—techniques for reducing CO2 in the oceans so they, in turn, can draw more CO2 from the air. Nevertheless, just before the heat wave began, I offered to help organize a virtual panel discussion on mCDR for an ocean research conference. Most of the questions that arose during the session were about fears that such research could create a moral hazard, allowing people to claim that drawing down CO2 lessens the urgency of reducing fossil-fuel emissions.
During the panel, a First Nations scholar from the University of British Columbia, Candis Callison, talked about how to involve local shoreline communities where ocean field trials might be conducted. Callison was a brilliant voice, helping the scientists understand more about public discourse on climate change. Just days later wildfires flared up and damaged several First Nations reserves—including the home, Callison informed me, where her relatives lived. This tragic event vividly reminded me of the dangers we already faced after one degree C of global warming. The tragedies could become much worse, given that even optimistic scenarios indicated the world would warm by at least another degree. I decided mCDR research was important. If it ultimately showed that the methods were futile or hazardous, the research could prevent prolonged investment in a false hope. If the work revealed safe ways to stimulate the ocean to take up more CO2, then those could be new tools to help stabilize the climate.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Starting in the 1950s, scientists began analyzing air bubbles trapped in ice cores drilled from the Greenland and Antarctic ice sheets to understand climate history. By the 1980s they realized that the world’s oceans could inhale or exhale enough CO2 to substantially contribute to Earth’s long-term cycles of ice-sheet expansion and retreat across continents. The leading hypothesis at that time for the seesawing of carbon concentrations in the ocean over thousands of years was that the surface water contained iron, which blew in from arid landscapes during cold periods, and its levels regulated phytoplankton growth across the seas. More iron would cause more growth, which would pull more CO2 from the air. Oceanographer John Martin of Moss Landing Marine Laboratories in California proposed that artificially fertilizing the ocean with iron could influence climate. At a 1988 meeting at the Woods Hole Oceanographic Institution in Massachusetts, Martin voiced what would become one of the most memorable quotes in oceanography: “Give me half a tanker of iron, and I will give you an ice age.”
Martin’s iron hypothesis prompted more than a dozen artificial iron-enrichment experiments between 1992 and 2009. Researchers released iron on the ocean’s surface and tracked for days or weeks how the area’s water chemistry and organisms changed. Results confirmed that iron enrichment could lead to a phytoplankton bloom when other conditions were favorable. Whether or not oceanographers considered these experiments “geoengineering,” the studies yielded extraordinary insight into the interacting biological and chemical processes that could alter climate on long timescales.
Serious concerns about interfering with nature grew, however, and nations worldwide signed a 2008 amendment to the London Convention. It prohibited further ocean-fertilization experiments beyond “small-scale, scientific research studies within coastal waters,” which chilled enthusiasm for such work. For the next decade researchers conducted studies mostly in virtual oceans, using models.
Several events started to thaw scientists’ cold views. The 2015 Paris Agreement’s goal of limiting warming to 1.5 degrees C set off a slew of studies about how much worse two degrees C of warming would be for ecosystems and for society. The research revealed that every fraction of a degree of avoided warming offers protection from serious dangers, including increasingly extreme heat, drought, and loss of terrestrial and marine biodiversity. Even though breakthroughs in renewable energy had slowed emissions’ tremendous rise, carbon removal would also be needed to stabilize the global climate.
Oceanographers are still debating how to prove whether any ocean strategy can effectively remove CO2 from the atmosphere.
Against this backdrop, the National Academies of Sciences, Engineering, and Medicine released a report on mCDR in 2022 that outlined six major strategies and the research required to evaluate them. The document provided social acceptance for marine scientists to pursue such work. Companies were looking for ways to buy credible carbon credits on a large scale, adding to the urgency. In 2022 financial services company Stripe and several large corporations committed to buying $1 billion of credits for carbon removal and permanent storage, on land and in the sea, to help guarantee demand that could accelerate the development of carbon-reduction technologies.
Progress has begun in earnest. The National Oceanic and Atmospheric Administration and the U.S. Department of Energy have both held competitions for proposals on mCDR science. Research is underway across the world. In June 2024 a company called Vesta spread 8,200 metric tons of crushed olivine—rock dust—on the ocean just offshore of North Carolina to try to absorb CO2 directly from the water. Vesta was the first company with a federal permit to test carbon removal from U.S. seas. Also in June, start-up Equatic began engineering, in Quebec, for a demonstration-scale plant that alters seawater chemistry to absorb more CO2.
Water at the ocean’s surface routinely exchanges gases with the atmosphere. Nitrogen, oxygen, CO2, and other trace gases each exert a part of the atmosphere’s overall pressure. In the ocean, CO2 also exerts a partial pressure, along with water and other molecules. When the partial pressure of CO2 in the ocean is lower than its partial pressure in the atmosphere, CO2 dissolves in seawater as wind pushes air against the waves. The air and water seek an equilibrium in their CO2 levels. As society’s carbon emissions intensify, the atmospheric CO2 partial pressure increases, and more of the gas is moved into the ocean. Most of the incoming CO2 reacts with seawater to form bicarbonate and carbonate, which—like salt in the ocean—remain dissolved in the water column for millennia. Since the industrial revolution more than 150 years ago, the ocean has absorbed approximately 25 percent of human CO2 emissions, a great service that has significantly slowed the pace of climate change.
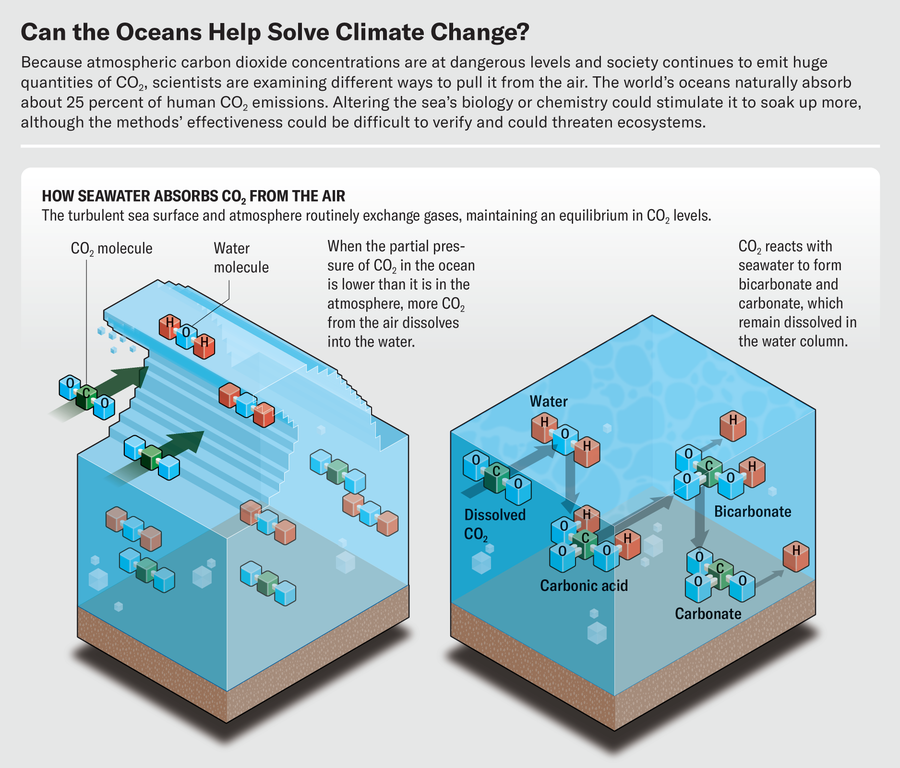
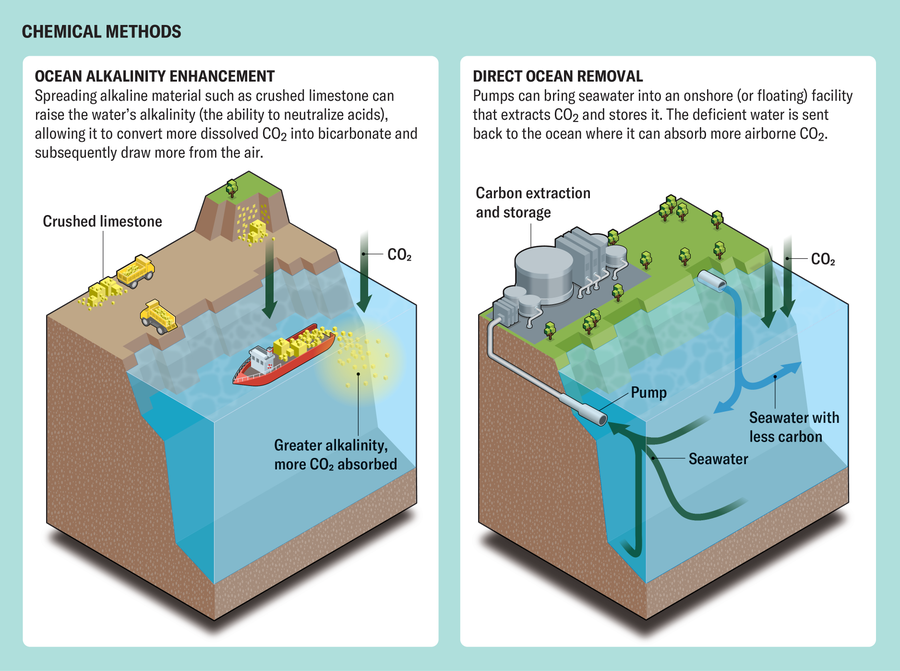
The goal of all the mCDR strategies is to lower the partial pressure of CO2 in the ocean’s surface layer, using either chemical or biological means. One category of chemical mCDR has the wonky name “ocean alkalinity enhancement.” Alkalinity is the water’s ability to neutralize acids. About 99 percent of the atmospheric CO2 the ocean absorbs becomes bicarbonate or carbonate, and this percentage can go higher when the water is more alkaline. Therefore, when an alkaline substance dissolves in seawater, the reaction reduces the partial pressure of CO2, allowing the water to absorb more from the air. Spreading pulverized alkaline rock such as limestone or olivine across the ocean or on beaches, as Vesta did in June, can raise alkalinity.
In a second category of chemical mCDR, called direct ocean removal, seawater is pumped into a floating or onshore facility that extracts CO2 and transports it for commercial use or stores it underground. The water is then pumped back into the sea, ready to absorb more CO2 from the air. Researchers are trying a variety of technologies to extract the carbon, such as electrodialysis, which forces water through a membrane, similar to how desalination plants operate. Captura is one company that is pursuing this approach.
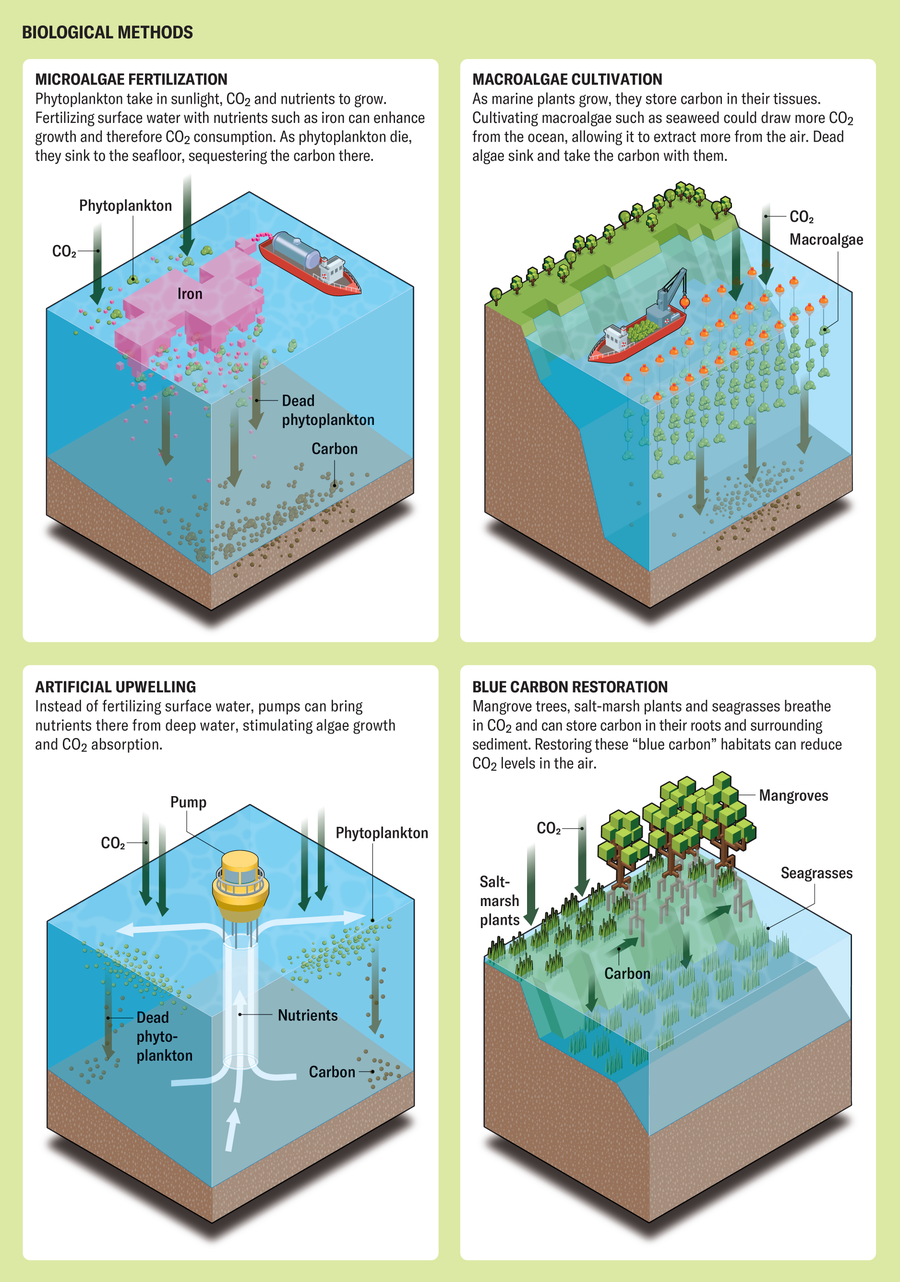
Biological techniques depend primarily on plant life, large and small. One approach is to cultivate macroalgae such as kelp—often compared with planting trees on land. As the plants grow, they store carbon in their tissues. For sequestration, the plants would be sunk to the seafloor, where the carbon might settle in sediment or remain dissolved in deep ocean waters.
Some scientists are continuing to experiment with the original fertilization approach to boost microalgae—the tiny, single-celled phytoplankton that are ubiquitous in the sea. Nutrients such as iron, phosphorus or nitrogen would be added to the sea surface, where they could prompt phytoplankton to photosynthesize and therefore grow faster than they otherwise would. The hope is that when these phytoplankton die and sink, the carbon stored within their cells will remain in the deep ocean. Another biological approach is artificial upwelling: pumps would move nutrient-rich water from the ocean’s interior toward the surface to try to boost macroalgae or microalgae growth.
Oceanographers are still debating how to prove whether any mCDR strategy can effectively remove CO2 from the atmosphere. With enough boats, sensors and people, we can measure the effect of an mCDR deployment on the surface water and then calculate the increased ocean uptake of CO2 while the effect is still detectable. For any longer period, we would need to rely on models because the water that was initially altered would be stirred by ocean currents over a wide region and diluted below the level of detectability. The ocean already stores 50 times as much CO2 as the atmosphere, but it is highly variable in space and time, so directly measuring the total additional carbon from an mCDR intervention is nearly impossible. Ironically, the same traits mean some mCDR methods could move a substantial amount of atmospheric CO2 into the ocean while barely perturbing the ocean’s background state.
The work to track these effects has become known as monitoring, reporting and verification. The goal is to try to monitor the carbon removed over time and report those results to a third party for independent verification. In direct ocean removal, for example, instruments at the extraction facility would measure the CO2 removed from seawater, and water samples collected from the nearby ocean, along with models, would track the additional carbon uptake at sea. Quantifying the carbon inside dead phytoplankton sinking to the sea bottom would be much harder.
Of course, rigorous monitoring, reporting and verification would also be needed if companies and organizations are to pay for carbon credits—say, $300 for a metric ton of sequestered CO2. The first issue is that we need to be sure the CO2 would not have been removed from the atmosphere without the intervention. We call this property an intervention’s additionality. For example, if kelp cultivation creates a thick mat of seaweed at the surface, but that growth creates a sunshade that slows photosynthesis in phytoplankton that would have lived a bit deeper, then the net change in ocean uptake of CO2—the additionality—might be zero.
The second issue is durability: how long captured carbon remains safely sequestered from the atmosphere. Phytoplankton enriched by iron might bloom, and a portion of their carbon-rich biomass may sink below the surface. But if microbes consume that carbon or it returns to its dissolved form, the carbon may again enter the atmosphere in a matter of months or years, lowering the durability of the intervention.
This past May I attended a meeting of scientists, engineers, philanthropists and government representatives to discuss every angle of ocean alkalinity enhancement. We eagerly scrutinized the first results from a field trial in Halifax Harbor off Nova Scotia, led by a company called Planetary Technologies and academic partners at Dalhousie University in Halifax. The trial involved water that a shoreline power plant was discharging into the harbor after it had been used for cooling, a routine practice. Researchers added alkaline compounds to the outflow pipe and measured the added alkalinity in the inner harbor. But because the signal was diluted beyond that area, they could only estimate the total carbon removal, using a tested ocean model. The early results suggest the trial successfully removed carbon that would have remained dissolved in the water without the work. And because that carbon is now stored in the ocean largely as bicarbonate, it should be stable for thousands of years—a highly durable result.
To assess additionality and durability in the vast and turbulent ocean, the research community will have to depend on a blend of direct observations and modeling because the complete impact of any mCDR deployment will play out over months or years. It will occur over areas of ocean too extensive to be directly observed and at levels too small to detect. A whole new generation of “applied ocean biogeochemists” is needed for this task.
Scientists must also assess whether a technique would endanger ecosystems or communities and weigh risks against any potential benefits. Large offshore kelp farms, for example, could disrupt local ecology or interfere with fisheries. For direct ocean carbon removal, companies would need plans to safely store or sell the CO2 produced as a waste product. Electrochemical technologies would filter and pump large volumes of seawater, and the machinery could suck in and destroy small plants and animals. Every approach has trade-offs, but so does leaving CO2 in the atmosphere to cause ongoing warming.
Researchers considering biological methods have the difficult challenge of proving that large-scale manipulation of the ecosystem is safe. The history of humans tinkering with ecosystems is littered with failures. Consider the importation of South American cane toads to Australia. The toads were brought to eat beetles feeding on sugar cane but became a toxic pest and national menace. It is difficult to predict every potential pitfall in open environments. Some of the basic problems we might look for when considering unintended consequences of biological mCDR are the accidental stimulation of harmful algal blooms that can poison shellfish, the expansion of oxygen-poor “dead zones” that can suffocate fish, and ecosystem effects on the food web, including fisheries.
Not emitting CO2 is the most reliable solution, but if the last 10 percent of reductions is difficult, ocean removal could help.
We would also have to assess whether biological methods pursued in one place could cause problems in another. If we spread iron on the ocean surface, more phytoplankton will also consume other nutrients they need to grow, such as nitrate and phosphate. Across the Southern Ocean encircling Antarctica, strong westerly winds create upwellings of vast nitrate and phosphate reservoirs from the deep ocean, which suggests that is where iron fertilization could be most effective. Ocean currents carry those upwelled nutrients across the globe, however, sustaining up to 75 percent of ocean photosynthesis in low latitudes. If iron fertilization prompts phytoplankton to consume these nutrients in the Southern Ocean, marine ecosystems throughout the rest of the world would be robbed of this nutrition—a serious ecological issue. And reduced growth worldwide would probably mean less CO2 drawn naturally from the air; in the end, there might be little additionality.
An mCDR technique that removes additional carbon, stores it durably and is safe must also pass one other test: Can it scale? The world emits more than 37 billion metric tons of CO2 annually. If we don’t want to bail out a sinking ship with a teacup, we should look for strategies that can remove about a billion metric tons a year. How might the options stack up?
Ocean alkalinity enhancement could hypothetically reach tens of billions of metric tons a year, if evaluated solely on the availability of appropriate rock that could be ground into alkaline powder. Logistically, however, it would be most efficient if the rock were spread by ships already sailing along existing maritime transport routes, and models suggest this approach would cut the potential to approximately one billion to three billion metric tons of CO2 drawdown per year.
Moreover, the mining and grinding of huge volumes of rock requires substantial energy and comes with its own social and ecosystem impacts on land. Nearly seven billion metric tons of limestone (an alkaline rock) are mined and crushed for agricultural and other applications every year, so the world would need a new industry equally as large to clean up a fraction of our CO2 pollution problem.
An evaluation of biological methods starts with the estimate that, worldwide, less than 10 billion metric tons of CO2 in the surface ocean naturally ends up sinking to the bottom every year within macroalgae and microalgae that die—deep enough to remain in the ocean for at least 100 years. To remove an additional billion tons of CO2 that won’t quickly reenter the atmosphere, a biological method would have to increase the total amount of biological material sinking into the planet’s deep seas by about 10 percent. It is hard to imagine that such a big increase would not have any major unintended consequences.
Answering the scaling question cannot be left to oceanographers alone. We must evaluate all the engineering, energy and economic challenges associated with deployment. Community engagement is needed to understand whether society is willing to interact with the ocean in this way. And, ultimately, if mCDR passes all the tests and has social acceptance, some entity or market would have to pay for it.
What can we reasonably ask of the ocean? The 37 billion metric tons of CO2 we emit every year constitutes a tiny percentage of Earth’s voluminous atmosphere, yet it has an outsize impact on our climate. Not emitting the gas in the first place is a far simpler, cheaper and more reliable solution than finding a technology to pull this trace gas out of the atmosphere.
But even if we could halve emissions within the coming decade and slash emissions by 90 percent just 20 years later, we will still face roughly 50–50 odds of overshooting 1.5 degrees C of global warming, the goal that almost every nation agreed to in the Paris Agreement. If the last 10 percent of emissions remained difficult to eliminate, warming would proceed indefinitely on a slower but still risky trajectory toward ice-sheet collapse and tens of meters of sea-level rise. We are researching mCDR so strategies might someday help society solve the final few percent of problems.
We must be honest when assessing promises and perils. At the Ocean Sciences Meeting—a leading conference of oceanographers from around the world—held last February in New Orleans, it seemed to me that everyone was talking about mCDR. Many scientists expressed cautious hope that further research might prove ocean alkalinity enhancement is safe and cost-effective; it is the most likely to be scalable and durable. Many researchers were skeptical that biological methods could be proved safe, verifiable or scalable. Still, with the need to slow climate change feeling increasingly urgent and given how much these studies might teach us about the ocean itself, interest in further research remains high.
Engaging in mCDR research requires a tremendous amount of hope. The techniques matter only if all our other efforts to mitigate climate change—from renewable energy to more walkable cities—reduce carbon emissions to a small fraction of what they are today. Only if we slow the gushing faucet of emissions to a trickle can mCDR possibly open the drain enough to stop the buildup of CO2 in the atmosphere.

































